Introduction
Björn Edwin
Laparoscopic liver surgery has progressively developed over the past two decades owing to its safety and efficacy.(1)

While in the 1990s laparoscopic liver surgery was considered mostly for benign lesions located in the anterolateral segments, nowadays an increasing number of patients undergo laparoscopic resection in the posterosuperior liver segments and laparoscopic major hepatectomies for primary liver cancer and metastases from different origin in specialized centers. (1-3)
The advantages of laparoscopic liver surgery over open surgery are well known and include smaller incision, less postoperative pain and reduced opioids requirement, shorter length of stay, and decreased postoperative complications.(3)
The first report in laparoscopic major hepatectomies by Huscher et al 4 published in 1997 reported perioperative results comparable to the open counterpart. Later in 2004, O’Rourke and Fielding (5), in their report of 12 laparoscopic right hepatectomies, demonstrated feasibility and safety of this procedure. Later, similar results were obtained by Dagher et al (6) in a multicentre study reporting 210 laparoscopic major liver resections (including mostly right hepatectomies) from six international surgical centers.
Recently published the first completed randomized controlled trial into laparoscopic and open parenchyma-sparing liver resection for colorectal liver metastases reported significantly less postoperative complications after laparoscopic resection and it was cost-effective than open resection. (7)
Despite the current success in laparoscopic liver surgery, laparoscopic major hepatectomies are limited to highly specialized centers and experienced surgeons. (1,8 ’9’10) The current level of evidence of laparoscopic major hepatectomy is restricted by retrospective studies and systematic reviews 8-10 . The multicenter randomized controlled trial the ORANGE 2 PLUS Trial into laparoscopic and open major hepatectomies is in the patients recruiting phase and will provide important evidence in this field.(12)
In the recently published first systematic review and meta-analysis using individual patients data on laparoscopic versus open major hepatectomy by M. Kasai et al (1-3) included a total of 917 patients (laparoscopic group-427, open group-490). In this report postoperative hospital stay was shorter, and morbidity was lower in the laparoscopic group, while operative time was shorter in the open group. Other perioperative outcomes (blood loss, perioperative mortality and blood transfusions) were similar, as well as the overall survival rates in the patients with colorectal metastases and hepatocellular carcinoma.
Laparoscopic right hepatectomy is considered to be more demanding than left hepatectomy (1) and associated with a high risk of injury to major vessels in the liver hilum, during dissection. This technically challenging procedure is limited to expert surgeons who have already acquired a large experience with easier laparoscopic liver resections.
Surgical technique
Patient’s position. The patient is placed in the 30-45 degree side with the right side up or in the supine position. The surgeon either stands between the patient’s legs or stands to the patient’s right side. Pneumoperitoneum is established by maintaining intraperitoneal pressure from 12 to 14 mm Hg which can be increased up to 19-20 mm Hg in case of bleeding.
Trocar placement. Number and position of trocars may vary from surgeon to surgeon, but 4 to 6 ports are necessary for this procedure.
Pringle maneuver. The Pringle manoeuvre (PM) can be used if the dissection of hilum and the parenchyma is expected to be difficult. It may be of value to prepare for a PM in case of risk of bleeding. There are different ways to prepare for PM.
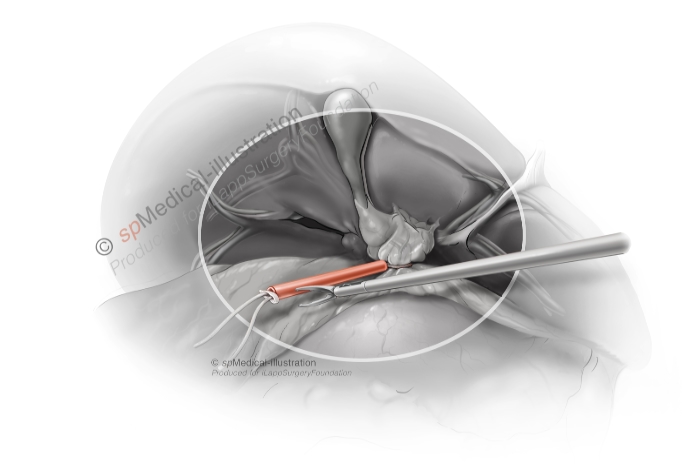
Intracorporeal Pringle manoeuvre
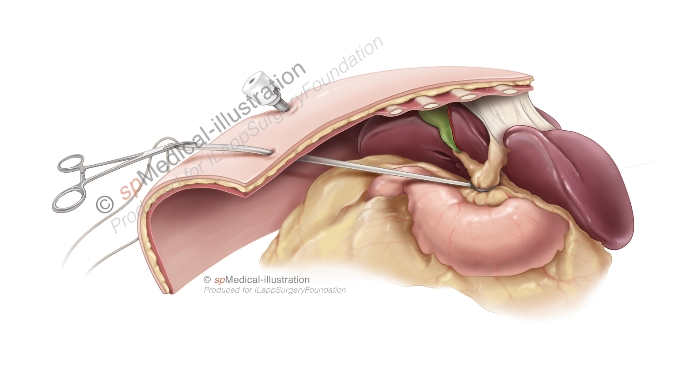
Extracorporeal Pringle manoeuvre
The main steps. The right hepatectomy may be divided into four main steps: liver mobilization, vascular inflow control, parenchymal transection and hepatic venous outflow control.
1. Liver mobilization
After the falciform ligament division, the liver is gently pushed downward using a retractor. The coronary ligament is divided until the suprahepatic vena cava and the right hepatic vein are exposed.

The right liver is then totally mobilized from ligamentous (coronary and triangular) attachments. The Makuuchi ligament is not necessary to dissect free and divide, because it will be divided in the last step with the endostapler. Short hepatic veins opening to the inferior vena cava are sealed / ligated individually using, before the transection of the parenchyma starts. It can be dangerous to use clips on these small vessels because the clips may fall off. Then to avoid rotation of the left lobe the round ligament shall be saved as well as to preserve the umbilical vein in cirrhotic patient.
2. Vascular inflow control
A. The classical extrahepatic approach:
Based on our experience we feel that this is the safest technique in our hands. Inflow vascular control starts with cholecystectomy. The gallbladder is externalized, but not divided from the main bile duct to use it as a handle to open the space to the portal trunk. The different structures in the ligament (artery and the right branch of vena porta) are dissected free and divided. First, right hepatic artery is identified, clipped and sectioned. The right portal vein is identified, mobilized and dissected after identifying portal bifurcation and the left portal vein. Both dissection and division can be done with different instruments; each surgeon has his/her own preferences. A small endo-swab can be useful when dissecting the vessels. During the portal vein dissection it is important to come in to the right layer as close as possible to the wall of the vein. It is important to place a vessel-loop around the right portal vein for the retraction and better access. Most used for division are clips (e.g. Hem-o-lok®) or endo-staplers. At this step surgeon should be aware of the small branches to the segment one. The division of the right hepatic bile duct are done within the hilar sheath during parenchymal transection, with an endo-stapler, in attempt to minimize the risk of damage to the left bile duct.
Sometimes the hilum can be very difficult both to reach and dissect, therefor other different approaches are useful to know about. It is important to know that Glissonian pedicle can be completely isolated both extrahepatically and intrahepatically.
B. Extrahepatic Glissonian approach or “isolation of the Glissonian pedicle”:
The Glissonian sheath is isolated, surrounded and divided extrahepatically with an endovascular stapler.
C. Intrahepatic Glissonian approach or transparenchymal clamping of the Glissonian pedicle:
The Glissonian sheath is isolated within the substance of the liver during parenchymal transection and secured using an endovascular stapling device
D. “Intrahepatic Glissonian approach”. Modified by Machado et al.
The Glissonian pedicle approach by incising the liver parenchyma on either side of the pedicle, thereby facilitating the introduction of a vascular clamp over the pedicle. The clamp can be replaced with an endovascular stapler and the pedicle can be divided.
The three last techniques has the advantage to be fast and being unlikely to cause injury to vascular structures and biliary drainage on the contralateral side. The hilar approach has been adopted by the majority of European specialized centers, while some centers outside Europe tend to apply a Glissonian approach.(1,14,1 5)
4) Hepatic venous outflow control
- After completed parenchyma transection right hepatic vein is dissected and divided with remaining ligament by an endo-stapler. We prefer to divide the right hepatic vein at the end of the operation as a last step. The dissection of the right hepatic vein before the right lobe is fully mobilised and “hanging over”, may be difficult and dangerous. In case of bleeding from this region, it can be almost impossible to handle it laparoscopically.
- Intra-operative ultrasound. Intra-operative ultrasound is used to define tumor location and its relation to main liver vessels, to indentify vessels in liver hilum and to control resection line.
The order of these steps may vary as it is described for caudal and anterior approaches. (16 ’17) The choice between the techniques depends on the surgeon’s preferences, tumor size and localization, and liver fragility. (1) Most surgeons will modify the technique in a way that makes them feel safe.